It's not sleep, nor the gloomy thought of the workday ahead, that makes us take it for granted that once joined, coffee and milk are inseparable. We know, even in the afternoon, that once poured, amen, it's gone. There's no going back. No one can give us back the coffee and milk we shared before. But the worst thing, which not even theoretical physicists in pajamas notice, is that following that simple act of pouring coffee into the cup with the hot milk, we increase the entropy of the entire universe. There's no need to worry, of course; it's not serious, but it's true! Since its discovery, the Second Law of Thermodynamics has established the existence of entropy as a function capable of quantifying irreversible processes and the disorder to which such processes tend, physically formalizing the fundamental principles of cause and effect and irreversibility. And the story of the latte is precisely one of the countless typical examples of the irreversibility we live with daily, and which gives meaning to our universe. Cause, effect, and irreversibility are, in fact, the three words that best describe the one-way nature of the reality around us, where everything seems to always be oriented in a precise direction, where causes always precede effects, and time always flows in a single direction. But are we really sure that this is always the case?
For example, if we take two closed chambers, one containing a certain amount of hydrogen and the other empty, and place them in contact with a thin tube, a certain
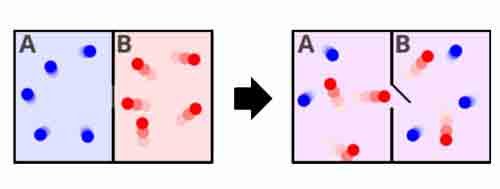
quantity of hydrogen molecules will pass from one container to the other depending on the thermodynamic parameters of the system (volume, pressure, temperature). At first glance, this process also seems completely irreversible, because the molecules that have permeated the second container will never spontaneously return to the first, just as the molecules of coffee and milk will never spontaneously separate in our cup, also because this would mean a decrease in entropy and an apparent violation of the Second Law of Thermodynamics. Henri Poincaré, however, rigorously demonstrated that this is not true for gas molecules, and that there are "recursions," or very long cycles at the end of which the molecules will find themselves in their initial state. In other words, entropy will most likely continue to increase, but sooner or later it will necessarily decrease to allow a return to the starting conditions. Note, however, that the cycles postulated by Poincaré are indeed enormously long, on the order of 10^N seconds, where N is the number of molecules in the system being considered (consider that a volume of just 40 dm^3 of air would have a cycle of about 10^24 seconds, which is the number of molecules contained in that volume, while the age of the universe is only 10^17 seconds!). It's a bit like imagining we keep shaking a puzzle box at random, hoping that the pieces will all fall into place and the picture will form automatically. Likewise, there's nothing preventing the hydrogen molecules in our two connected containers from all returning to the first container as in the initial condition. Except that such a situation, while perfectly possible like the puzzle, has a very low probability of occurring, and therefore we must wait an extremely long time before observing such an event.
And what about time, then? Could it be that, even in the case of time, there is a privileged direction simply because it is much more probable than the opposite? Perhaps, from our lazy human perspective, we take too many things for granted, when perhaps not even the existence of time itself is a given, but merely an illusion of us poor three-dimensional beings with extremely limited perspectives. The Universe, ever since we began to examine it from a quantum perspective, has continually told us that it is not exactly as we see it and believe it to be—quite the opposite! We still have a long way to go before we understand the intimate nature of Existence!


 quantity of hydrogen molecules will pass from one container to the other depending on the thermodynamic parameters of the system (volume, pressure, temperature). At first glance, this process also seems completely irreversible, because the molecules that have permeated the second container will never spontaneously return to the first, just as the molecules of coffee and milk will never spontaneously separate in our cup, also because this would mean a decrease in entropy and an apparent violation of the Second Law of Thermodynamics. Henri Poincaré, however, rigorously demonstrated that this is not true for gas molecules, and that there are "recursions," or very long cycles at the end of which the molecules will find themselves in their initial state. In other words, entropy will most likely continue to increase, but sooner or later it will necessarily decrease to allow a return to the starting conditions. Note, however, that the cycles postulated by Poincaré are indeed enormously long, on the order of 10^N seconds, where N is the number of molecules in the system being considered (consider that a volume of just 40 dm^3 of air would have a cycle of about 10^24 seconds, which is the number of molecules contained in that volume, while the age of the universe is only 10^17 seconds!). It's a bit like imagining we keep shaking a puzzle box at random, hoping that the pieces will all fall into place and the picture will form automatically. Likewise, there's nothing preventing the hydrogen molecules in our two connected containers from all returning to the first container as in the initial condition. Except that such a situation, while perfectly possible like the puzzle, has a very low probability of occurring, and therefore we must wait an extremely long time before observing such an event.
quantity of hydrogen molecules will pass from one container to the other depending on the thermodynamic parameters of the system (volume, pressure, temperature). At first glance, this process also seems completely irreversible, because the molecules that have permeated the second container will never spontaneously return to the first, just as the molecules of coffee and milk will never spontaneously separate in our cup, also because this would mean a decrease in entropy and an apparent violation of the Second Law of Thermodynamics. Henri Poincaré, however, rigorously demonstrated that this is not true for gas molecules, and that there are "recursions," or very long cycles at the end of which the molecules will find themselves in their initial state. In other words, entropy will most likely continue to increase, but sooner or later it will necessarily decrease to allow a return to the starting conditions. Note, however, that the cycles postulated by Poincaré are indeed enormously long, on the order of 10^N seconds, where N is the number of molecules in the system being considered (consider that a volume of just 40 dm^3 of air would have a cycle of about 10^24 seconds, which is the number of molecules contained in that volume, while the age of the universe is only 10^17 seconds!). It's a bit like imagining we keep shaking a puzzle box at random, hoping that the pieces will all fall into place and the picture will form automatically. Likewise, there's nothing preventing the hydrogen molecules in our two connected containers from all returning to the first container as in the initial condition. Except that such a situation, while perfectly possible like the puzzle, has a very low probability of occurring, and therefore we must wait an extremely long time before observing such an event.
